Navigating Legal and Ethical Challenges in High Throughput Screening for Drug Discovery

What is High Throughput Screening in Drug Discovery? A Detailed Introduction
High Throughput Screening (HTS) is a method used in drug discovery to rapidly test large numbers of drug compounds against specific biological targets. The goal is to identify potential drug candidates that can act on these targets, speeding up the early phases of drug development. HTS allows researchers to screen thousands, sometimes even millions, of compounds quickly, which would be impossible using traditional methods. This process helps identify “hits” — compounds that show some level of biological activity — that can be further refined into drug leads or candidates for clinical trials.
HTS plays a critical role in drug discovery by reducing the time and cost involved in the initial stages of the process. Rather than manually testing compounds one by one, automation and robotics in HTS systems allow for the screening of large libraries of compounds at once. This efficiency helps pharmaceutical companies and research institutions accelerate the discovery of new treatments, especially in areas like cancer, infectious diseases, and neurological disorders.
The Role of HTS in Drug Discovery
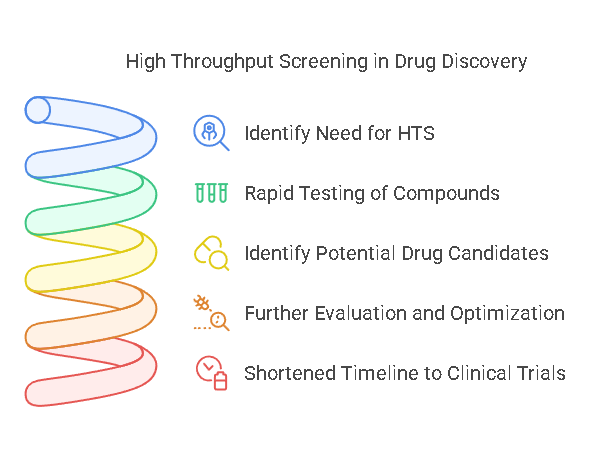
High Throughput Screening is an essential tool in drug discovery because it accelerates the identification of potential drug candidates. By enabling rapid testing of large compound libraries, HTS helps researchers focus on the most promising candidates early in the development process. These identified candidates, also known as “hits,” can then undergo further evaluation and optimization, such as lead discovery, lead optimization, and preclinical testing.
HTS significantly shortens the timeline from the discovery phase to clinical trials, giving researchers more opportunities to identify druggable targets and evaluate their therapeutic potential. It has revolutionized the way drugs are discovered by increasing efficiency, cutting costs, and ensuring that research efforts are focused on the most promising compounds.
How Does HTS Work?
HTS involves several key steps that enable researchers to efficiently test thousands of drug candidates. These steps typically include:
- Compound Library: HTS begins with a large library of diverse chemical compounds. These libraries can contain thousands to millions of compounds that have been preselected for their potential to interact with specific biological targets.
- Automation: One of the key aspects of HTS is its use of automation. Robotic systems are used to handle and prepare samples, ensuring that the screening process can be carried out in an efficient and consistent manner.
- Assays: HTS relies on assays to test the biological activity of the compounds. There are two primary types of assays used in HTS: biochemical assays and cell-based assays. Biochemical assays test the direct interaction between the drug candidate and the biological target, while cell-based assays assess how the compound affects living cells.
- Data Analysis: After the compounds are tested, data analysis tools are used to evaluate the results. Sophisticated software systems analyze the data to identify “hits” — compounds that show biological activity against the target of interest.
- Hit Identification and Validation: Once hits are identified, they are validated through secondary assays to confirm their activity. These validated hits can then move on to further development, where they are optimized for potency, selectivity, and safety.
HTS systems are often integrated with advanced technologies like miniaturized assays (which allow for smaller sample volumes), and data analytics (which enables the extraction of meaningful information from large datasets).
Benefits of HTS in Drug Discovery
High Throughput Screening offers several significant benefits that contribute to the overall success of drug discovery:
- Cost-Effectiveness: HTS reduces the need for manual screening, saving both time and money. By automating the process and allowing large numbers of compounds to be tested simultaneously, HTS cuts down on labor costs and increases the throughput of drug discovery programs.
- Time Savings: Traditionally, testing individual compounds can take months or even years. HTS allows for the testing of thousands of compounds in a matter of days or weeks, significantly accelerating the early drug discovery process.
- Scalability: HTS is highly scalable, meaning that as new compound libraries become available, the screening process can expand to include them without additional manual labor. This scalability is crucial for the continuous discovery of new drug candidates.
- Improved Drug Candidate Identification: HTS enables the discovery of compounds that might not have been identified using traditional screening methods. By testing a diverse library of compounds, HTS can uncover novel drug leads for diseases that have limited treatment options, such as rare or orphan diseases.
- Targeting Specific Disease Mechanisms: HTS enables the discovery of drugs that target specific disease mechanisms. For example, researchers can identify compounds that interact with certain proteins involved in cancer cell growth, enabling the development of targeted therapies.
- Enhanced Drug Development in Various Therapeutic Areas: HTS has proven especially useful in oncology, infectious disease research, and the development of therapies for neurological conditions. Its ability to rapidly identify compounds makes it ideal for addressing complex diseases that have long been difficult to treat.